


First and Best-in-Class Breakthrough Oral Therapies
to Reverse Retinal Neurodegenerative Diseases

Composed of talented experts with solid proven track records in drug development, clinical research and management at multinational pharmaceutical companies.



| - | Grant from Innosuisse, Swiss Federal Innovation Agency -- Over 1 million CHF in total. |
| - | Grant from SME Instrument of EU Horizontal 2020 Program, Phase 1 supported 50K EURO. |
| - | Grant from Baselaunch, a Swiss biopharma Incubator provided funding, office and other resources. |
| - | Winner of the Johnson & Johnson sponsored Startup Slam competition at the 2018 BIO-Europe. |
| - | Winner of MAGINE IF Innovation Forum. |
| - | Approved by J&J to become a residence of JLABS. |

◦ Mr. Hong Yu
◦ Mr. William Johnson
◦ Mr. David Guan
◦ Mr. William Johnson
◦ Mr. Hong Yu
◦ Mr. David Guan
◦ Mr. Hong Yu
◦ Mr. William Johnson
◦ Dr. Yuhong Dong



Retinitis Pigmentosa (RP)
The first sign of RP is usually a loss of night vision, which becomes apparent in childhood. Problems with night vision can make it difficult to navigate in low light. Later, the disease causes blind spots to develop in the side (peripheral) vision. Over time, these blind spots merge to produce tunnel vision. The disease progresses over years or decades to affect central vision, which is needed for detailed tasks such as reading, driving, and recognizing faces. In adulthood, many people with retinitis pigmentosa become legally blind. Currently, there are approximately 2 million RP patients worldwide.
The typical clinical phenomenon of RP indication is apoptosis of photoreceptor cells, which leads to visual impairment and blindness. SunRegen is developing first-in-class drugs to prevent and repair photoreceptor apoptosis through an anti-neuronal apoptosis and neuroprotective mechanism, thereby protecting and repairing visual function.
Dry Age-related Macular Disease (DAMD)
DAMD is a common eye disorder among people over 50. It causes blurred or reduced central vision due to the breaking down of the inner layers of the macula. The macula is the part of the retina that gives the eye clear vision in the direct line of sight.
DAMD is a slow deterioration of the cells of the macula, often over many years, as the retinal cells die off and are not renewed. The term ‘dry’ does not mean the person has dry eyes, just that the condition is not wet AMD. As of 2024, approximately 200 million people worldwide are affected by Age-related Macular Degeneration (AMD). This number is projected to increase to 288 million by 2040 due to aging populations, and 85-90% of AMD patients are DAMD.
There is no effective treatment for DAMD. Existing treatments can only improve the symptoms and slow down the disease but cannot completely cure DAMD.
SunRegen is developing innovative drug to prevent and repair photoreceptor apoptosis through an anti-neuronal apoptotic mechanism and improve visual function by enhancing lysosomal scavenger protein aggregation and reducing lipofuscin accumulation.
Optic Atrophy (OA)
Optic atrophy is a disease that affects the function of the optic nerve, resulting in lesions of retinal ganglion cells and their axons, and clinically classified into two main categories: primary and secondary optic atrophy.
Primary Optic Atrophy (POA) is an optic neuropathy that primarily affects the ganglion cells and their axons between the retina and the lateral geniculate body. The main symptoms of primary optic atrophy include reduced vision and a grayish-white or pale optic disc. When the nerve fiber layer around the optic disc is damaged, slit-like or wedge-shaped defects may occur. The number of small blood vessels in the optic disc may be reduced, and the retinal arteries may become thinned and narrowed, or even occluded.
Secondary Optic Atrophy (SOA) is a manifestation of optic nerve damage, which unlike primary optic atrophy, is usually caused by other eye diseases or lesions. Clinically, it is commonly seen in secondary optic atrophy caused by diseases such as glaucoma and diabetic retinopathy. Glaucoma Optic Atrophy (GOA) is very common among later stage glaucoma.
According to Globaldata, there are currently nearly 2 million patients in the United States, China, Japan and 5 Western Europe countries.

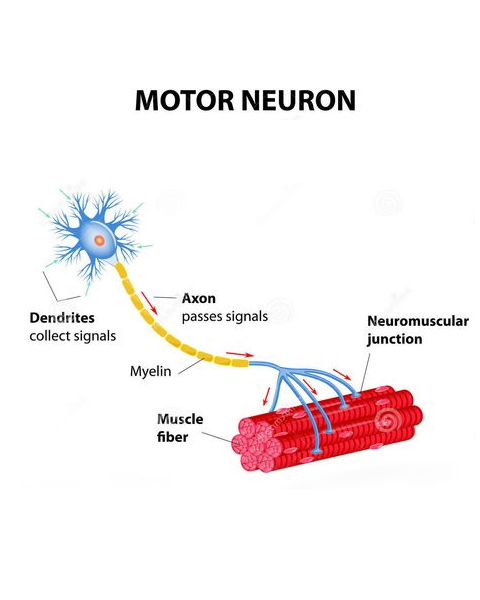
Central Nerve System Neurodegenerative Diseases
Neurodegenerative diseases are characterized by a progressive deterioration of central and peripheral neural functions resulting from evolving pathological insults to neurons and/or glial cells in brain, spinal cord and peripheral nerves, leading to neuron death. Neurodegenerative diseases have a major impact on patients at a professional, social and family level and can lead to a complete inability to carry out any type of everyday activity. For example, patients may have mobility problems, breathing difficulties, cognitive problems, or gradual memory loss.
Neurodegenerative diseases are growing into a global challenge, as medical advances ensure more individuals live longer. Worldwide, at least 55 million people suffering from Alzheimer’s disease or other dementias. If breakthroughs are not discovered, patient number would exceed 152 million by 2050. All current therapies for neurodegenerative diseases aim to relieve symptoms and do not target the process of neuron death.


SunRegen is focused on the development of neurodegenerative related drugs. Currently, we are focusing on the development of our lead compound, SBC003, for the treatment of neuronal apoptosis-related diseases, starting with retinal neurodegenerative diseases and then expanding to the treatment of CNS neurodegenerative diseases.


1 September 2023

11 April 2019


20 June 2018

1 March 2018

3 October, 2017
25 August 2017

21 August 2017

10 August 2017

22 June 2017
| - | One Compound for Multiple Indications |
| - | Easily Accepted Oral Dosage |
| - | Synthetic Compound with Cost Effective Production |
| - | Neuroprotective and Neuro-rescuing Treatment Effects in 5 Species, 10 Toxins, and 20+ Models |
| - | Excellent In Vivo Safety |
| - | Passes Blood Brain Barrier & Blood-Retinal Barrier |
| - | Converging Data from Models of 10+ Neurological Diseases |
| - | One Blind Patient Successfully Treated with SBC003 Precursor |
| - | Breakthrough Therapy, Orphan Drug & Fast Track Designation |
| - | With unprecedented breakthrough discovery, SBC003 is expected to be the first, best and epoch-making oral medication to reverse retinal neurodegenerative diseases. FDA is expected to provide up to 7 years of market exclusivity for SBC003 as an orphan drug. |
| - | Projected annual peak sales of SBC003 is very promising. Although the first indication RP is a rare disease, the expected annual peak sales could exceed $1.3 billion USD (Blockbuster). The projected annual peak sales of SBC003 to treat and reverse major retinal neurodegenerative diseases including DAMD and OA could exceed $60 billion USD. |

SunRegen Healthcare AG
Business Parc Reinach
Christoph Merian-Ring 11
4153 Reinach
Switzerland